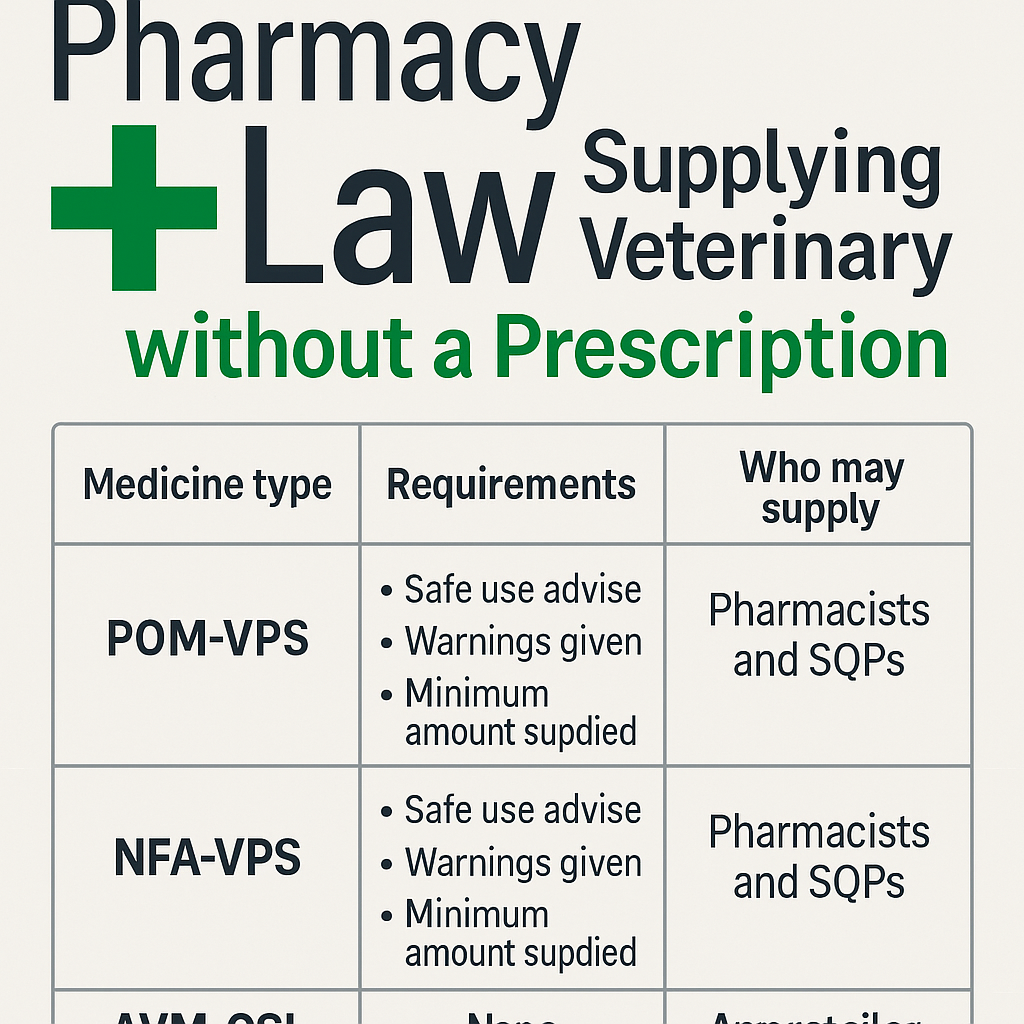
Supplying Veterinary Medicines Without a Prescription (UK Law)
Under the Veterinary Medicines Regulations 2013 (VMR 2013) and Veterinary Medicines Directorate (VMD) guidance, veterinary medicines fall into four legal categories with specific supply rules: Category Prescription Needed? Who May Supply Legal Reference POM‑V Yes – only a vet Veterinary surgeon VMR 2013 Sch 3 para 3(2–3) POM‑VPS May be supplied without prescription if prescriber and supplier are […]